Introduction: Acute chest syndrome (ACS) is one of the leading causes of mortality in patients with sickle cell disease (SCD). Despite this, there are no biomarkers that can distinguish patients who present with a painful vaso-occlusive episode (VOE) and may rapidly develop ACS. Endothelial damage is a critical component of the pathophysiology of SCD. Small extracellular vesicles (EVs) are endocytic-derived vesicles that package protein and nucleic acid cargo for communication between cells. We and others have demonstrated that EVs have unique properties in patients with SCD. We have shown that EVs from SCD patients differentially affect endothelial integrity in vitro. We have previously performed miRNA sequencing and identified 15 miRNA that were differentially expressed based on a history of ACS. In this study we sought to closely assess how three of these miRNA, miR-let7c-5p, miR-122-5p, and miR-369-5p, differ across the timeline of patients with sickle cell, ie. at baseline, during ACS and during VOE.
Methods: The SCD biobank at the University of Chicago Comer Children's Hospital and LaRabida Children's Hospital was queried for patients who had multiple blood samples over many years that included at least one each of a baseline (> 4 weeks from an acute event), a hospitalization for ACS and a hospitalization for a VOE. All subjects had been prospectively enrolled, with informed consent provided by parents or patients aged >18 years and assent obtained from subjects aged 9-18 years. Control samples (n=5) were obtained from patients without SCD who received bloodwork for routine check-ups or for follow-up of iron-deficiency anemia. Equal volumes of control samples were pooled for consistency across experiments. EVs were isolated via Total Exosome Isolation Kit (Thermo Fisher Scientific) per the manufacturer's guidelines. RNA was purified from samples using the miRNeasy Micro Kit (Qiagen). For quantitative PCR, complementary DNA was generated with the TaqMan Advanced miRNA cDNA Synthesis kit (Thermo Fisher Scientific). Specified miRNAs (hsa-miR-122-5p, hsa-miR-99a-5p, hsa-miR-369-5p, hsa-miR-let-7c-5p) were analyzed using TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific) and TaqMan Advanced miRNA Assay primers (Thermo Fisher Scientific). Samples were analyzed in triplicate on a 7500 Fast Real-Time PCR machine (Applied Biosystems) and threshold cycle values were normalized to cel-miR-39 controls. Relative changes in expression for ACS and VOE samples were calculated using the respective baseline samples or non-SCD controls as reference values and theΔΔ quantification cycle method.
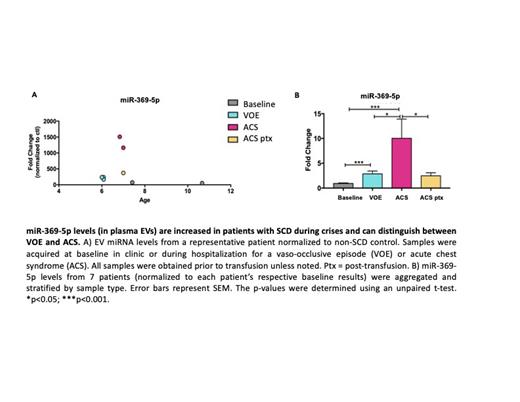
Results: To determine whether exosomal miRNA levels correlated with the disease status of patients, normalized samples from all patients were aggregated and then stratified based on sample type (baseline, VOE, ACS, or ACS post transfusion). While there were no statistically significant differences in exosomal expression of miR-let-7c-5p and miR-122-5p between sample types, there was a promising trend for miR-122-5p. There was a 2.6-fold increase in miR-122-5plevels, relative to baseline, when patients experienced a vaso-occlusive episode. In contrast, miR-122-5p levels decreased 2.3-fold, relative to baseline, when patients were hospitalized with acute chest syndrome. Extravesicular miR-369-5p levels were differentially regulated and correlated with the disease status of patients. When patients were hospitalized with a VOE or ACS, there was a 2.9-fold and 10-fold increase in miR-369-5p levels, respectively, compared to their baseline. In addition, there was a statistically significant difference between VOE and ACS samples, which suggests that miR-369-5p levels can distinguish between VOE and ACS events (Fig. 3C). Interestingly, when ACS samples were obtained post-transfusion (ACS ptx), miR-369-5p levels decreased significantly to values near that of VOE samples.
Conclusions: In this study, we report that the miRNA-369-5p levels in small EVs are differentially up-regulated in SCD patients during an ACS event. Furthermore, we show that these levels can distinguish patients presenting with a VOE from those who eventually develop ACS. Our findings demonstrate that it is possible to utilize extracellular vesicles and their contents to predict ACS. Further studies should be done so there is a tool to predict ACS and improve the clinical outcomes for patients with SCD.
Disclosures
No relevant conflicts of interest to declare.